Digital Pills : Ingestible Sensors are Changing Medication
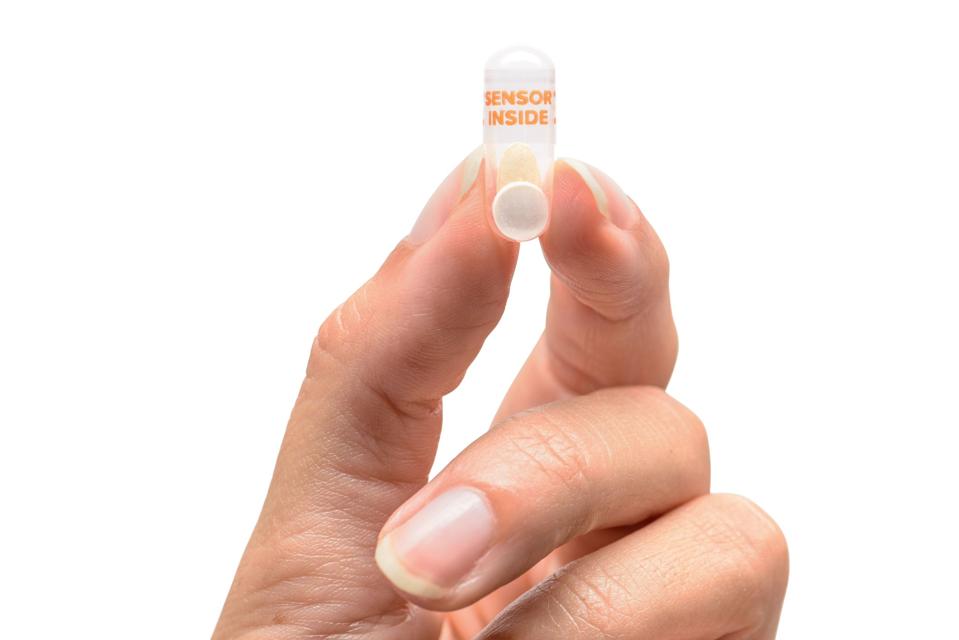
Monika Pandey – Imagine swallowing a pill that can tell your doctor whether you actually took your medication. This isn’t science fiction it’s the reality of modern healthcare. Digital pills with ingestible sensors represent one of the most significant advancements in pharmaceutical technology, creating a new paradigm for managing treatment compliance. These innovative medications contain tiny, safe-to-ingest sensors that activate upon contact with stomach fluid, transmitting data to external devices and healthcare providers. The emergence of this technology addresses one of medicine’s most persistent challenges: the fact that approximately 50% of patients with chronic conditions don’t take their medications as prescribed. This breakthrough has profound implications for clinical trials, chronic disease management, and mental health treatment, potentially saving billions in healthcare costs and countless lives.
Understanding how digital pills function requires exploring their unique composition and data transmission process. These medications combine traditional pharmaceuticals with miniaturized technology designed to survive the harsh environment of the human digestive system.
A complete digital pill ecosystem consists of several interconnected elements that work seamlessly together:
An ingestible sensor made of dietary minerals that activates upon contact with stomach fluid
A small wearable patch that detects the sensor’s signal and records activity data
Mobile applications that display medication-taking patterns and adherence trends
Secure cloud-based platforms where healthcare providers can access patient data
Alert systems that notify caregivers when doses are missed
Integration capabilities with electronic health records for comprehensive care coordination
From ingestion to data delivery, the digital pill follows a sophisticated pathway that transforms medication administration into a measurable event:
The patient swallows the medication containing the embedded sensor
Stomach fluids activate the sensor, which begins transmitting a unique signal
A wearable patch on the patient’s torso detects this transmission
The patch records the time and date of ingestion alongside physiological data
Information transfers securely to a mobile device via Bluetooth technology
Data uploads to encrypted cloud servers accessible to authorized healthcare providers
Algorithms analyze patterns to identify potential adherence issues
Patients receive reminders and encouragement through the mobile application
The applications for digital pills span numerous medical specialties, offering particular promise for conditions where treatment compliance directly impacts outcomes and costs.
Pharmaceutical research stands to gain tremendously from objective medication adherence data:
Researchers obtain accurate, real-time information about participant compliance
Clinical trial results become more reliable by eliminating adherence uncertainty
Drug efficacy assessments improve with verified dosing information
Safety monitoring enhances through correlation of effects with actual ingestion times
Regulatory approvals may accelerate with superior adherence documentation
Placebo effects can be better distinguished from true pharmacological actions
Patients with complicated medication schedules benefit from the supportive nature of digital monitoring:
Elderly patients with multiple chronic conditions receive automated adherence support
Transplant recipients maintain crucial immunosuppressant regimens with oversight
Tuberculosis patients complete full courses of treatment under remote supervision
HIV-positive individuals sustain antiretroviral therapy with discreet monitoring
Hypertension sufferers optimize blood pressure control through verified medication intake
Diabetic patients coordinate multiple medications with integrated tracking systems
While the technology offers significant advantages, its implementation involves addressing several practical aspects that affect both patients and healthcare systems.
The sensitive nature of medication adherence data necessitates robust protection protocols:
All transmitted information undergoes end-to-end encryption
Patients maintain control over who accesses their adherence data
Healthcare providers access information through secure, HIPAA-compliant platforms
Data anonymization protects patient identity during research and analysis
Clear consent processes ensure patient understanding of data usage
Regular security audits maintain system integrity against potential breaches
Integrating digital pill technology requires thoughtful consideration of systemic factors:
Insurance coverage and reimbursement policies need adaptation for these advanced medications
Healthcare provider education ensures appropriate patient selection and monitoring
Pharmacy systems must update to handle distribution and tracking of digital medications
Technical support structures assist patients with device usage and troubleshooting
Clinical workflows incorporate adherence data review into standard care practices
Outcome studies continue to demonstrate the technology’s value across different populations
Digital pill technology continues to evolve, with ongoing research and development expanding its potential applications and refining its capabilities.
The next generation of digital medicines promises even greater integration with healthcare:
Combination with physiological sensors to correlate medication intake with vital signs
Miniaturization of components for easier swallowing and greater patient comfort
Extended battery life for wearable sensors enabling longer monitoring periods
Artificial intelligence algorithms that predict and prevent adherence lapses
Integration with smart home devices for seamless medication management ecosystems
Development for pediatric populations with appropriate consent and monitoring protocols
As with any emerging technology, digital pills face hurdles that require thoughtful solutions:
Cost considerations must be balanced against potential savings from improved outcomes
Technological literacy barriers necessitate user-friendly designs for diverse populations
Regulatory frameworks continue to evolve around data privacy and appropriate use
Long-term reliability studies will further establish the technology’s durability
Cultural acceptance of medication monitoring requires education about benefits
Equity in access must be ensured across socioeconomic and demographic groups
This website uses cookies.